Which Element Has the Following Configuration Xe 6s24f15d1
To work backwards and find the element with a given configuration you first find the noble gas in the Periodic Table. Which element has the following configuration.
Can Somebody Explain In Details How Ce Can Have The Electron Configuration Xe 6s24f15d1 Quora
Experts are tested by Chegg as specialists in their subject area.
. 2 Which element has the following configuration. 1 What is the ground-state electron configuration of the oxide ion O2. Express your answer in condensed form in order of increasing orbital energy.
Rn7s 2 5f 14 6d 10 7p 6 note Notes on the Electron Configuration of particular elements. The electron configuration of an atom tells us how many electrons are in each orbital. Which element has the following configuration.
The chemical symbol for Xenon is Xe. Expert Answer 96 24 ratings The chemical symbol of the element is Cerium ie. Thus the electron configuration of Mn2 would be Ar3d5.
For example helium has two electrons in the 1s orbital. You have been given the number of electrons which for the neutral element is necessarily the same as the number of. For example He2s22p2 should be entered as He2s22p2.
What is the ground-state electron configuration of a. The element is defined by Z the atomic number which is the number of protons positively charged massive nuclear particles. See the answer Which element has the following configuration.
Although the electron configuration of Cerium Ce is Xe6s24f15d1 and the max energized orbital is 5d1 but it is the only element having one electron in 4f orbital 4f1. Who are the experts. We make electonic cpnfiguration according to bulidingup principle and hunds rule.
Therefore the electron configuration of He is 1s2. It must be element 56. The electronic configuration is there to distract you.
The electron configuration of an atom tells us how many electrons are in each orbital. Cerium has an electron configuration Xe6s24f15d1 and lutetium ends the lanthanide series with Xe6s2 4f145d1. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Which element has the electron configuration xe 6s2 4f14. When forming ions the transitions metals lose their outermost s-electrons before losing any d-electrons. According to Madelung rule which describes the electronic configuration and filling up of el.
From the periodic table Xe has atomic number 54. Illustratively the max energized orbital for the element Al is 3p1 so for the value 3p1 Al is assigned from periodic table. Write the condensed electron configurations for the following atoms using the appropriate noble-gas core abbreviations.
The actinides follow the same configuration as the lanthanides. If you have any questions please visit the link below. Z5421410282 and therefore the element is LEAD.
View this answer Xe6s24f15d1 X e 6 s 2 4 f 1 5 d 1 means that the element to be identified has the configuration of Xenon plus electrons in the 6s 4f and 5d shell. What is the chemical symbol for xenon. Value is a guess based on periodic table trend.
Xe is element 54. What element has a noble gas notation Xe 6s2. Thus Ce is assigned to 4f1.
Simple way to get to this answer is to count the number of electron or protons in the given configuration. Therefore the electron configuration of He is 1s2. Together 5425 61.
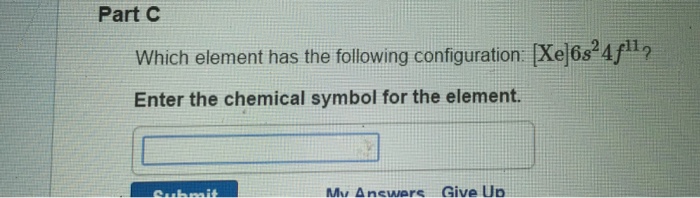
Enter the chemical symbol for the element. The element with atomic number 61 is Promethium Pm which is a Lanthanide. Xe6 s 24 f 15 d 1.
Your element has two more electrons and two more protons than Xe. 6s orbital has 2 electron. Xe has 54 electron.
Xe6s 2 4f 3. Electron Configurations of Atoms and Ions. Enter the chemical symbol for the element.
Answer 1 of 2. For example helium has two electrons in the 1 s orbital. And 4f orbital has 5 electron.
Rn7s 2 5f 14 6d 10 7p 5 note Praseodymium. We add the departure electrons which are now a total of 82. It glows lovely Slytherin house green color P.
View the full answer. We review their content and use your feedback to keep the quality high. For the element having an electron configuration of Xe 6s2 4f14 5d10 6p2 we need to know the atomic number Xe.

Solved Which Element Has The Following Configuration Chegg Com
Can Somebody Explain In Details How Ce Can Have The Electron Configuration Xe 6s24f15d1 Quora
Can Somebody Explain In Details How Ce Can Have The Electron Configuration Xe 6s24f15d1 Quora
No comments for "Which Element Has the Following Configuration Xe 6s24f15d1"
Post a Comment